Clinical engineering is a branch of biomedical engineering for professionals responsible for the management of medical equipment in a hospital. The tasks of a clinical engineer are typically the acquisition and management of medical device inventory, supervising biomedical engineering technicians (BMETs), ensuring that safety and regulatory issues are taken into consideration and serving as a technological consultant for any issues in a hospital where medical devices are concerned. Clinical engineers work closely with the IT department and medical physicists.
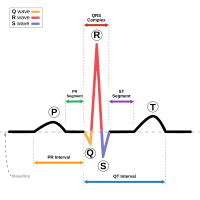 ythm, an example of a biomedical engineering application of electronic engineering to electrophysiology and medical diagnosis.
ythm, an example of a biomedical engineering application of electronic engineering to electrophysiology and medical diagnosis.A typical biomedical engineering department does the corrective aClinical engineeringnd preventive maintenance on the medical devices used by the hospital, except for those covered by a warranty or maintenance agreement with an external company. All newly acquired equipment is also fully tested. That is, every line of software is executed, or every possible setting is exercised and verified. Most devices are intentionally simplified in some way to make the testing process less expensive, yet accurate. Many biomedical devices need to be sterilized. This creates a unique set of problems, since most sterilization techniques can cause damage to machinery and materials. Most medical devices are either inherently safe, or have added devices and systems so that they can sense their failure and shut down into an unusable, thus very safe state. A typical, basic requirement is that no single failure should cause the therapy to become unsafe at any point during its life-cycle. See safety engineering for a discussion of the procedures used to design safe systems.

No comments:
Post a Comment